
(Jakarta, November 13, 2025)
Daewoong, in collaboration with Indonesia’s National Agency of Drug and Food Control (BPOM) and leading medical experts, held the “Anti-Counterfeit Media Briefing 2025”, highlighting the dangers of distributing unlicensed botulinum toxin and emphasizing the importance of using certified products.
The event is part of Daewoong’s ongoing Authenticity Certification Campaign in Indonesia, which promotes safe pharmaceutical distribution practices and strengthens patient protection through close collaboration with BPOM.
In his opening remarks, Baik In Hyun, Head of Daewoong Indonesia Business Unit, stated, “This campaign stems from our deep commitment to patient safety and the importance of building healthcare professionals’ trust. Despite our cooperation with BPOM to curb illegal online sales, unauthorized distribution and exposure through academic forums continue to occur.”
He added, “Daewoong’s botulinum toxin is the first high-purity product in Asia approved by the U.S. FDA and is now distributed in over 80 countries. Through this authenticity campaign, we are committed to building a safe and trusted distribution system together with Indonesia’s medical community.”
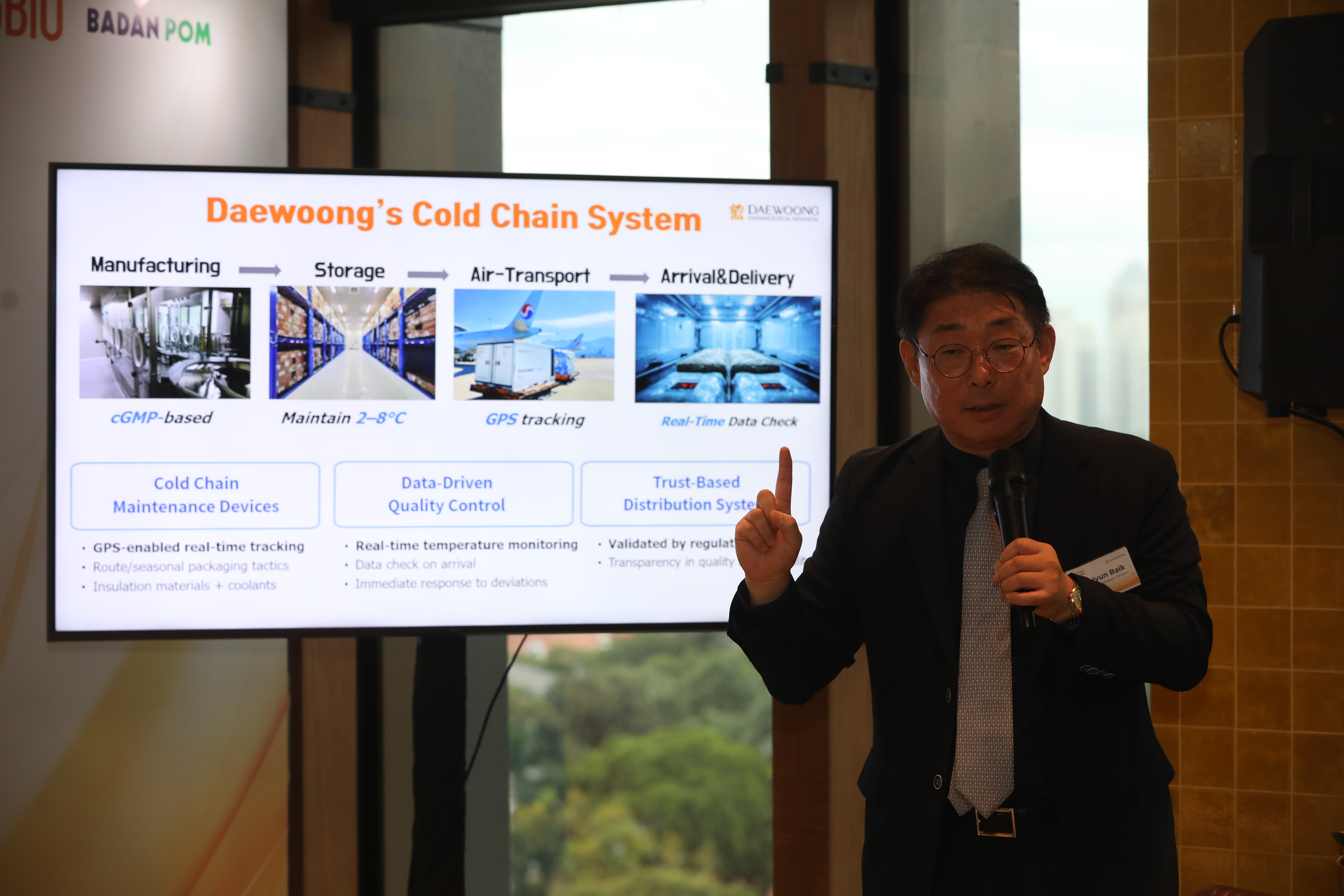
Daewoong implements comprehensive quality control across all stages of production, storage, and distribution of its botulinum toxin products, based on a high-technology cold chain system that maintains a stable temperature between 2–8°C.
For exports to Indonesia, Daewoong applies an air-shipment-based cold chain strategy meticulously designed to ensure far more consistent temperature stability compared to long-term sea transport. The packaging is also optimized for local climate conditions.
Through pre-validation processes, season- and route-specific packaging strategies, and post-arrival data verification, the company minimizes potential quality fluctuations. All containers are equipped with special insulation, cooling materials, and GPS tracking devices for real-time temperature monitoring.
If an anomaly occurs, a rapid reshipment system is immediately activated to maintain product integrity. Shipping data are communicated transparently to partners and relevant authorities to ensure supply chain reliability and objectivity.
With this transparent and credible system, Daewoong’s botulinum toxin products are now stably supplied to more than 80 countries worldwide.
At the same event, Head of BPOM RI Prof. Dr. Taruna Ikrar, M.Biomed., M.D., Ph.D., emphasized the government’s firm stance against illegal drug distribution, “BPOM guarantees the safety, quality, and efficacy of all circulating products. Therefore, every drug must have a distribution permit and meet established standards. Circulating unlicensed products is a violation that endangers patients, and BPOM — together with law enforcement — will take firm action against all forms of illegal distribution to protect the public and compliant industries.”

He added that the government not only regulates but also conducts direct enforcement and tracking of distribution routes in coordination with law enforcement and related agencies. He stressed that the purchase, storage, or use of drugs without a valid distribution permit constitutes a violation of the Health Law (Law No. 17 of 2023), and that medical professionals are not exempt from legal consequences for using illegal products.

The illegal distribution of unlicensed botulinum toxin violates several Indonesian laws and carries severe criminal penalties. First, importing, distributing, or using products without BPOM authorization violates Articles 138, 143(1), and 435 of the Health Law No. 17 of 2023 and Government Regulation No. 28 of 2024, punishable by up to 12 years in prison or a fine of up to IDR 5 billion.
Second, storing or using unregistered pharmaceutical products is classified as an illegal act under Article 2 of BPOM Regulation No. 27 of 2022, with criminal, civil, and administrative sanctions applicable to medical professionals as well.
Third, distributing or providing products without issuing tax invoices or transaction reports violates Value-Added Tax, Income Tax, and Customs laws, leading to administrative and additional criminal penalties.
Prof. Taruna reiterated, “This is not merely an ethical issue. Such illegal practices undermine the credibility of the entire medical industry and endanger public health. We hope this event marks a turning point for Indonesia in building a more ethical and safer medical ecosystem.

Meanwhile, Dr. Anesia Tania, a certified dermatologist, highlighted the risks of cold chain failure for botulinum toxin safety, “Biological substances like botulinum toxin lose their effectiveness and safety when the cold chain is broken. In clinical practice, we still encounter side effects and retreatment cases caused by products entering through unofficial channels.”

She added, “This authenticity certification campaign functions as a protective system, helping clinics and patients identify genuine products. Medical professionals must also uphold their professional responsibility to ensure patient safety.”
