
(Jakarta, Indonesia — December 24, 2025)
Daewoong Pharmaceutical (hereinafter “Daewoong”) announced on December 24th that it has officially obtained marketing authorization from Indonesia’s National Agency of Drug and Food Control (BPOM) for its new diabetes drug Enavogliflozin.
Indonesia ranks fifth globally in the number of adult (aged 20–79) living with diabetes. According to the International Diabetes Federation (IDF) Diabetes Atlas 2024, the number of adults in Indonesia currently living with diabetes is approximately 20.4 million, and is projected to increase to around 28.6 million by 2045, indicating a rapidly growing long-term disease burden.
Enavogliflozin, which has now received marketing authorization, is Korea’s 36th novel drug. Administered once daily at a low dose of 0.3mg, it demonstrated blood glucose-lowering efficacy compared to the existing SGLT-2 inhibitor Dapagliflozin in a Phase 3 clinical trial. In particular, clinical studies showed that once-daily Enavogliflozin achieved blood sugar control comparable to that of Dapagliflozin, with improvements in HbA1c and fasting plasma glucose. The safety findings from these studies support the potential for long-term use in clinical practice.
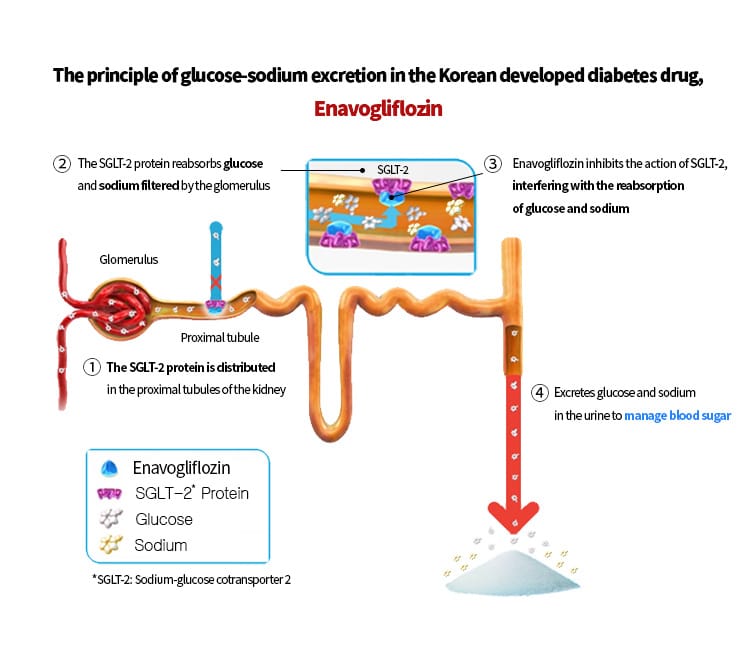
SGLT-2 inhibitors work by preventing the reabsorption of glucose and sodium in the kidneys via the sodium-glucose cotransporter 2 (SGLT-2) protein, leading to their excretion in urine and thereby lowering blood glucose levels. In addition to lowering blood sugar, these medicines are associated with modest weight loss and reduced blood pressure. Together, these effects may help support heart and kidney health while lowering the risk of diabetes-related complications.
Daewoong plans to go beyond simply obtaining marketing approval, using Enavogliflozin as a catalyst to expand academic exchange on diabetes treatment strategies between Korea and Indonesia, and to serve as a bridge for clinical insight sharing among medical professionals from both countries.
As part of these efforts, Daewoong Pharmaceutical Indonesia participated in the ‘Jakarta Diabetic Meeting 2025’ held in November, where the clinical value of the drug was introduced. In collaboration with major institutions such as the Indonesian Society of Endocrinology (PERKENI), the Indonesian Society of Internal Medicine (PAPDI), and the Faculty of Medicine, University of Indonesia (FKUI), Daewoong engaged with around 500 local healthcare professionals. In the same month, Prof. So Hun Kim, Professor of Endocrinology at Inha University College of Medicine Korea, gave a special lecture at the RSCM Kanigara Advanced Diabetes Center, where, together with Dr. dr. Tri Juli Edi Tarigan, Sp.PD-KEMD, FINASIM, Head of Endocrinology Division at Universitas Indonesia, discussed the diabetes epidemiology and treatment challenges of both countries, laying the groundwork for practical academic exchange through on-site education.
Following this marketing authorization, Daewoong plans to conduct an additional Phase 3 trial aimed at expanding the scope of use of Enavogliflozin. The goal is to build clinical evidence reflecting the characteristics of Indonesian patients and real-world clinical settings, to strengthen trust among healthcare professionals, and contribute to expanding practical treatment options.
Meanwhile, Daewoong is gradually establishing treatment options covering both diabetes and dyslipidemia to support the treatment of metabolic diseases in Indonesia. The approval of Enavogliflozin is part of this effort to expand its chronic disease treatment portfolio. In November, Daewoong launched dyslipidemia treatments in Indonesia, including the country’s first low-dose Ezetimibe-Rosuvastatin 10/5 mg fixed-dose combination, along with 10/10 mg and 10/20 mg variants, enabling tailored prescriptions based on patients’ individual cardiovascular risk.
Baik In Hyun, Executive Director of Indonesia Business Division at Daewoong Pharmaceutical Indonesia stated, “Enavogliflozin demonstrates Daewoong’s commitment to providing clinically proven, Asia-originated innovative treatments to Indonesian patients,” and added, “Based on its proven efficacy, excellent safety, and once-daily dosing convenience, we aim to offer evidence-based long-term diabetes management options to healthcare providers.”
He continued, “We will continue to strengthen academic collaborations with local societies and universities such as PERKENI and FKUI, and expand our cardiometabolic portfolio from dyslipidemia to diabetes, becoming a healthcare partner contributing to the health of the Indonesian people.”