%20v2.jpg)
7 Out of 10 Suffer from GERD… ‘Fexuprazan’ Gains Attention for Rapid and NighttimeRelief
- Updated GERD treatmentguidelines in Indonesia… highlight P-CAB drugs as a new therapeutic option
- Fexuprazan offers faster symptom relief and superior dosingconvenience, regardless of meals
- Indonesian clinical trials prove Fexuprazan's rapid effectiveness,nighttime symptom control, and high safety profile
(Jakarta,May 20, 2025)
Gastroesophageal reflux disease(GERD) is on the rise in Indonesia. According to a study by the University ofIndonesia’s Faculty of Medicine (Journal of Clinical Gastroenterology, April2024), GERD prevalence among Indonesian adults increased from 61.8% in 2019 to67.9% in 2021, affecting nearly 7 out of 10 individuals. The Westernization ofdietary habits, chronic stress, obesity, and aging populations are cited asmajor factors contributing to this increase.
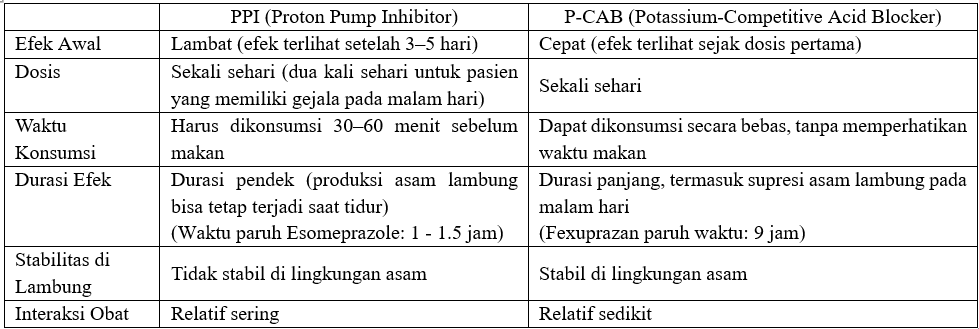
Daewoong Pharmaceutical, SouthKorea’s leading healthcare group, is drawing attention for developingFexuprazan, a new treatment for GERD, which has recently demonstrated itsefficacy and safety in clinical trials conducted among Indonesian patients. AlthoughPPIs are typically taken once a day, their short half-life often requirespatients to take them twice daily to control nighttime acid secretion, leadingto inconvenience. In contrast, Fexuprazan, with the longest half-life amongP-CAB drugs (approximately 9 hours), provides sufficient acid suppressionthroughout the night with just a once-daily dose, offering greater dosingconvenience, cost-effectiveness, and superior control of nocturnal heartburnsymptoms compared to PPIs.
◆Rising GERD Cases Lead to New Treatment Options with P-CABs
Historically, proton pumpinhibitors (PPIs) have been the first-line treatment for GERD. First introducedabout 40 years ago, proton pump inhibitors (PPIs) are absorbed into the body inan inactive form and become activated only in the acidic environment of thestomach. Once activated, they bind to the final stage of acid production—theproton pump—to exert their effect.
Because of this complex activationprocess, PPIs do not provide immediate relief after ingestion. They must betaken 30 to 60 minutes before meals, as activation requires the stomach to bein an acidic state. Moreover, PPIs can only act on already activated protonpumps. Since new pumps are continuously produced in the stomach, it typicallytakes 3 to 5 days of consistent use to see noticeable effects. With a shorthalf-life of just 1 to 2 hours, the duration of action is limited, and thedrugs are often insufficient to suppress acid production during the night. As aresult, some patients continue to experience nighttime heartburn, leading todisrupted sleep and reduced quality of life. Although PPIs are generallyprescribed once daily, many patients end up taking them twice a day foradequate symptom control.
Fexuprazan is a next-generationP-CAB drug designed to overcome these limitations of PPIs. It is absorbed in analready active form and does not require an acidic environment for activation,allowing it to act immediately upon ingestion. It can also be taken regardlessof meals, offering greater dosing flexibility and convenience.
Fexuprazan competitively binds tothe potassium (K⁺) binding site of the proton pump, the final gateway ofgastric acid secretion. By doing so, it blocks the exchange of potassium andhydrogen ions, directly inhibiting the acid secretion process at its source. Inaddition, Fexuprazan maintains high chemical stability even in highly acidicenvironments. It is not easily degraded by acid, allowing it to remain in thestomach for an extended period and deliver sustained acid suppression.
With a half-life of approximately 9hours — the longest among P-CAB drugs — Fexuprazan provides effective acidcontrol throughout the night with just a once-daily dose. As a result, itserves as a particularly effective treatment option for patients experiencingnighttime heartburn and sleep disturbances.
Recognizing the potential of thisnew class of therapy, the Indonesian Society of Gastroenterology (PGI)officially updated the 2024 GERD Treatment Guidelines to include P-CABs as arecommended treatment option alongside PPIs. This marks the first time P-CABshave been incorporated into Indonesia’s national clinical guidelines,reflecting growing confidence in their efficacy. In Japan as well, P-CABs arenow recommended as a first-line treatment for severe GERD, and consideredalongside PPIs for mild to moderate cases, reflecting the growing internationalrecognition and adoption of P-CAB therapies.
◆ FexuprazanDemonstrates Fast Symptom Relief in Indonesian Clinical Trials
Recent investigator-initiatedtrials in Indonesia confirmed Fexuprazan’s comparable efficacy to Esomeprazolewhile delivering faster symptom relief, particularly in alleviating nausea.
While patients on Esomeprazoletypically reported symptom improvement after eight weeks, those treated withFexuprazan showed significant improvement within just seven days. Additionally,within just seven days of treatment, patients receiving Fexuprazan showedsignificantly greater improvement in quality of life compared to the controlgroup, along with faster relief from nighttime reflux symptoms and improvedsleep quality.
Professor Ari Fahrial Syam, Chairof the Executive Board of the Indonesian Society of Gastroenterology andPrincipal Investigator of the study, stated, “This study confirmed thatFexuprazan alleviates heartburn and acid reflux symptoms faster thanEsomeprazole. Given the rising prevalence of GERD in Indonesia, Fexuprazan,which provides symptom relief with just once-daily dosing, represents aninnovative treatment option.”
Jisun Lee,Head of Clinical Development at Daewoong Pharmaceutical, stated,"Fexuprazan’s rapid symptom relief and dosing convenience position it as avaluable option to improve patients' quality of life in Indonesia. We arecommitted to continuously providing high-quality treatment options thatcontribute to the health of the Indonesian people."
Based on these positive clinicalresults, Daewoong Pharmaceutical is actively progressing with the regulatoryapproval process for Fexuprazan in Indonesia. The company also plans to expandits indications to include broader gastrointestinal conditions such asfunctional dyspepsia and gastritis, while strengthening collaboration withlocal medical societies and academic institutions.
%2520v2_4342976.jpeg)
- ## -
Ferry Maulana Prateja
Public Relations Supervisor
Daewoong Group Indonesia
+62 857 1839 7889
ferry.prateja@daewoong.co.kr